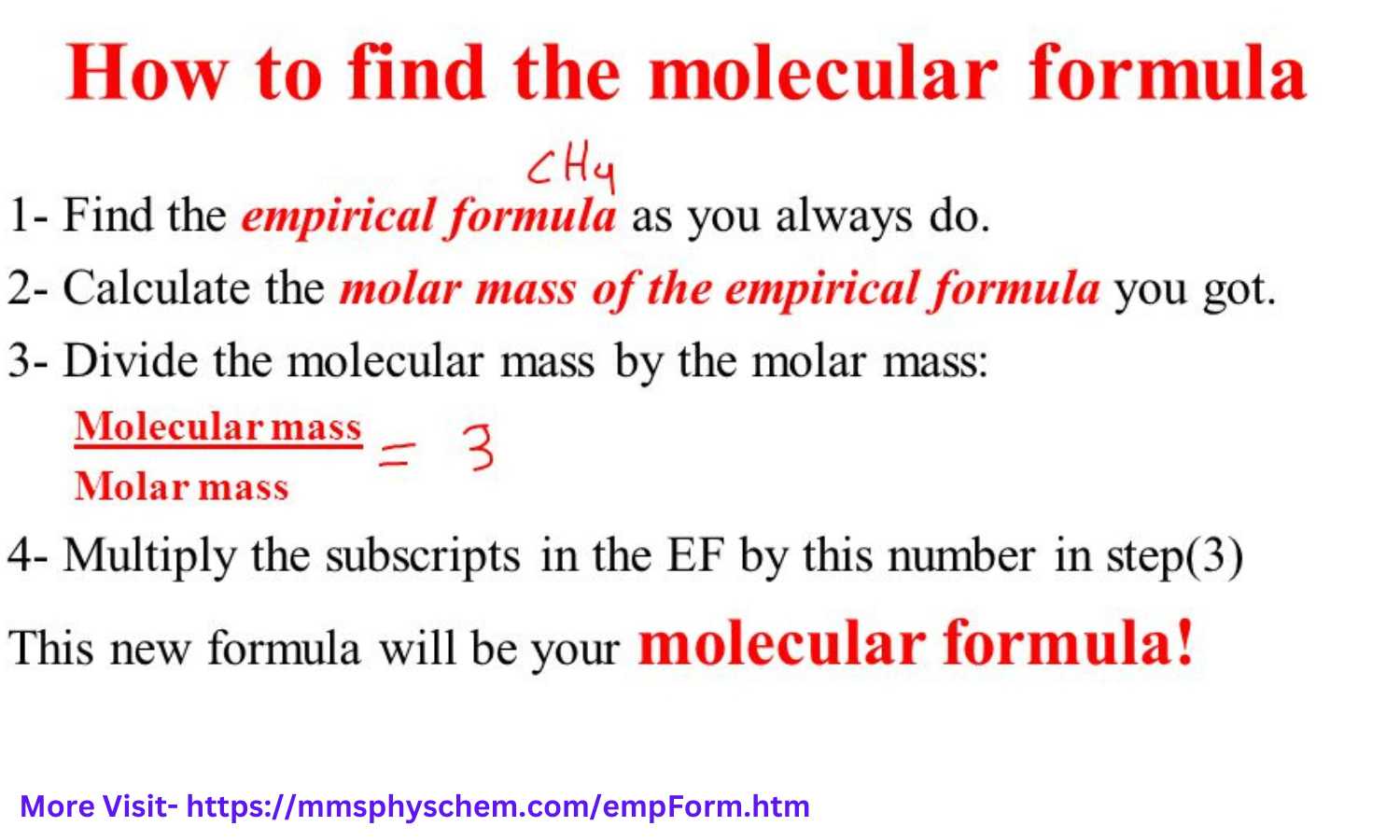
For example, consider a compound composed of 40% carbon, 6.67% hydrogen, and 53.33% oxygen by mass. Calculating the molar mass of each element and the empirical formula's molar mass can help determine whether the molecular formula needs adjusting to account for these proportions accurately.
More Visit- https://mmsphyschem.com/empForm.htm