Hirsutism or the growth of excessive male-pattern hair in women is a common endocrine disorder that impacts a number of women globally. Primarily affecting facial areas and areas of the public region, buttocks, and thighs, hirsutism results from increased androgen levels due to factors like obesity, pcod, and ovarian tumors; once evident it
Vaniqa is a topical application available in cream form for working on facial hair removal in hirsutism women. Using Eflornithine as its active ingredient, Vaniqa works on the ornithine decarboxylase enzyme to slow hair growth and reduce the growth intensity significantly. Manufactured by Allergan, Inc. and sold under Ornidyl in the States, Vaniqa is currently under the World Health Organisation's list of Essential Medications because of its therapeutic potential and multiple benefits.
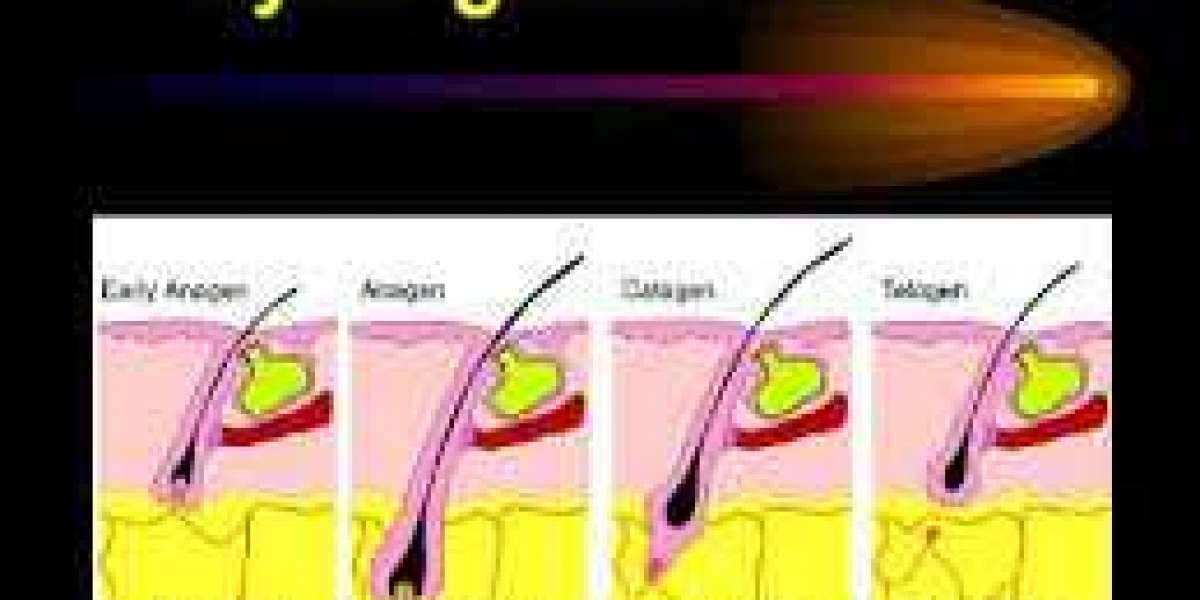
Eflornithine, the active ingredient of Vaniqa prevents hair growth by inhibiting the anagen phase of hair production and irreversibly binding to ornithine decarboxylase enzyme. The use of Vaniqa inhibits the growth phase and promotes the shedding phase of facial hair, in turn leading to a decrease in facial hair intensity.
A number of concerns about the safety of Vaniqa on the skin led researchers to conduct animal studies to study the possibility of cancerous lesions on the use of the cream. Photocarcinogenesis is a complex multistage process of tumor growth and development involving three distinct stages exemplified by the initiation, promotion, and progression of carcinogens.
In animal studies conducted for over twelve months to evaluate the possibility of In a 12-month photo carcinogenicity study in hairless albino mice, animals treated with the vehicle alone showed an increased incidence of skin tumors induced by exposure to ultraviolet light, whereas mice treated topically with eflornithine cream buy online at doses up to 600 mg/kg showed an incidence of skin tumors equivalent to untreated control animals. A two-year dermal carcinogenicity study in CD-1 mice with VANIQA revealed no evidence of carcinogenicity at daily doses up to 600 mg/kg. In this study, daily doses resulted in systemic exposures 200- to 1000-fold higher than systemic exposures following topical application in humans. At maximal doses, eflornithine did not elicit mutagenic effects in an Ames reverse-mutation assay or clastogenic in primary human lymphocytes, with and without metabolic activation. A dermal micronucleus assay at doses up to 900 mg/kg in rats also yielded no evidence of genotoxicity photo carcinogenicity with the use of Vaniqa cream
In dermal teratology studies, no maternal or fetal toxicity or teratogenic effects were observed in rats at doses up to 450 mg/kg or in rabbits at doses up to 90 mg/kg. Higher doses in both studies resulted in maternal and fetal toxicity without evidence of teratogenicity. Other Studies involving the use of Eflornithine topical 15% cream showed non-phototoxic and non-sensitizing reactions in guinea pigs.
The sum total of animal studies led researchers to come to the conclusion that Vaniqa is safe for use and can be used without the risk of photocarcinogenesis. But further human studies are required to arrive at a concrete conclusion as to its long-term safety on the human dermis. Most importantly, its use should be avoided in pregnant women for fetus safety rather than skin safety.
When considering whether or not to use Vaniqa, it is important to speak with your doctor first. They will be able to advise you on whether or not this medication is right for you and discuss any potential side effects. If you decide to use Vaniqa, it is available by prescription from your doctor or dermatologist. You can also purchase it online from various retailers, but make sure you do so from a reputable source.